- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- 3-O-Ethyl-L-ascorbic acid
- 86404-04-8
- C<sub>8</sub>H<sub>12</sub>O<sub>6</sub>
- 204.18
Your Location:Home > Products > Food Additives > 3-O-Ethyl-L-ascorbic acid
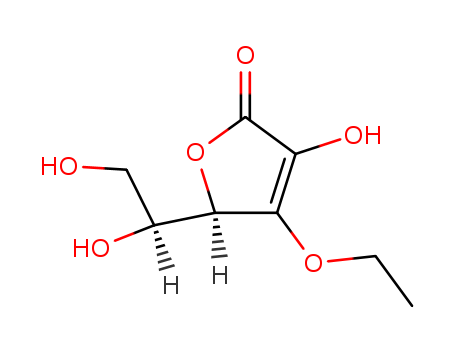


|
Mechanism of action |
VC ethyl ether penetrates the stratum corneum to reach the melanocytes in the basal layer, inhibits tyrosinase activity, inhibits the formation of melanin, and restores melanin to colorless, thereby effectively whitening the skin. And lookchem and VC ethyl ether can directly participate in the synthesis of collagen and repair skin cell activity after entering the dermis, increasing collagen, so that the skin becomes full and elastic, making the skin delicate and smooth. |
|
Synthesis |
The above 3-O-ethyl-5,6-O-isopropylideneascorbic acid 40g dissolved in 200ml of ethanol. 10g of cation-exchange resin was added and was refluxed for 2 hours. The catalyst was removed by an ion exchange resin and concentrated under reduced pressure. After drying, with ethyl acetate: hexane (3: 1) crystallization. After drying, obtained 33.3g 3-O-ethylascorbic acid, yield 94%. It was recrystallized in 100mL ethyl acetate giving 30.6g, yield 87%, mp: 112-114°C. |
|
Consumer Uses |
This substance is used in the following products: cosmetics and personal care products. Other release to the environment of this substance is likely to occur from: indoor use as processing aid and outdoor use as processing aid. |
InChI:InChI=1/C8H12O6/c1-2-13-7-5(11)8(12)14-6(7)4(10)3-9/h4,6,9-11H,2-3H2,1H3/t4-,6+/m0/s1
A single-step synthesis of 3-O-ethyl-L-a...
3 - O - Alkyl - ascorbic acid. 3 - O -al...
The invention discloses a vitamin C ethy...
The invention provides a method for synt...
The present invention relates to the fie...
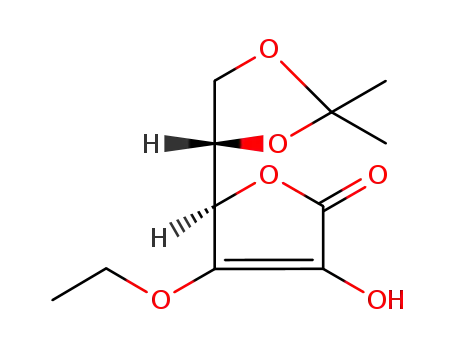
(R)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-4-ethoxy-3-hydroxyfuran-2(5H)-one

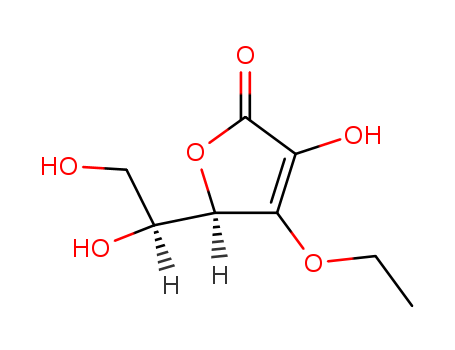
3‐O‐ethyl‐L‐ascorbic acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; at 60 ℃; for 3h;
|
98% |
|
With hydrogenchloride; In methanol; water; at 60 ℃; for 3h;
|
98% |
|
With cation-exchange resin; In ethanol; for 2h; Reflux;
|
87% |
|
With hydrogenchloride; water; at 60 ℃; for 2h; Product distribution / selectivity;
|
84.3% |
|
With hydrogenchloride; In propan-1-ol; at 60 ℃; for 2h;
|
56% |
|
With hydrogenchloride; Nafion H; water; In ethanol; at 60 ℃; for 2h; not specified Product distribution / selectivity;
|
|
|
With water; In ethanol; at 60 ℃; for 2h; Product distribution / selectivity;
|
|
|
With hydrogenchloride; In methanol; water; at 20 ℃; for 16h;
|
|
|
With toluene-4-sulfonic acid; In ethanol; at 60 ℃; for 2h; Solvent;
|
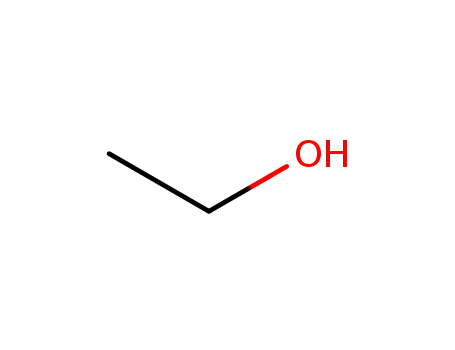
ethanol

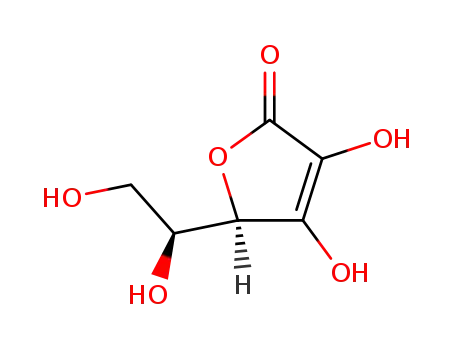
ascorbic acid


3‐O‐ethyl‐L‐ascorbic acid
| Conditions | Yield |
|---|---|
|
ascorbic acid; With tetrabutylammomium bromide; tin(IV) chloride; In ethyl acetate; at 40 ℃;
ethanol; With triethylamine; Diethyl carbonate; at 45 ℃; for 5h;
With hydrogenchloride; In water; at 55 ℃; pH=2; Temperature; pH-value;
|
76% |

(R)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-4-ethoxy-3-hydroxyfuran-2(5H)-one
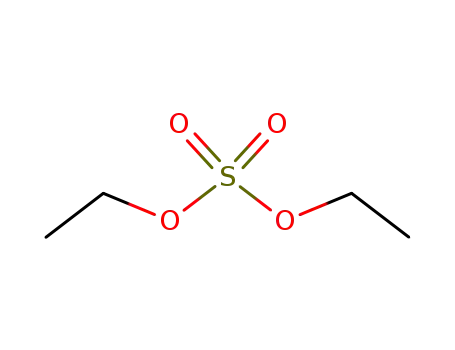
diethyl sulfate

ascorbic acid
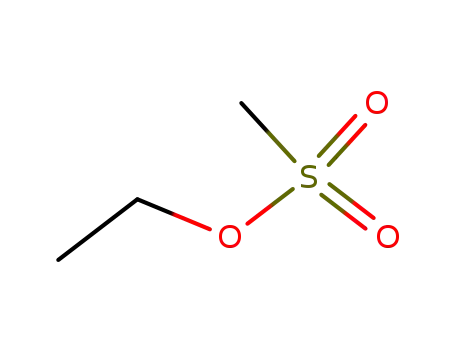
Ethyl methanesulfonate
