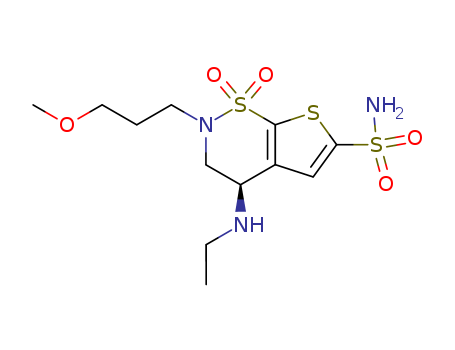
- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Brinzolamide
- 138890-62-7
- C<sub>12</sub>H<sub>21</sub>N<sub>3</sub>O<sub>5</sub>S<sub>3</sub>
- 383.514
- crystalline solid
Your Location:Home > Products > Biochemical Engineering > Brinzolamide


|
Preparation |
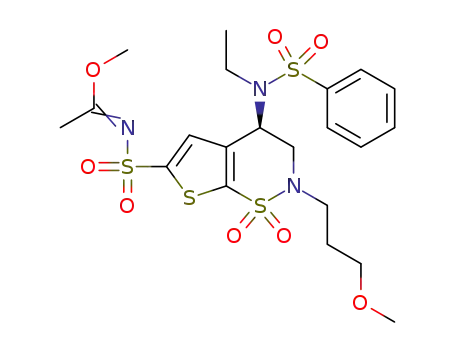
Brinzolamide synthesis method: using thiophene as raw material, 3-acetyl-2,5-dichlorothiophene (4) is obtained by chlorination and acetylation, and 4 is reacted with sodium benzyl sulfide to obtain 6,6, which is chlorinated and ammoniated. Chemical and oxidation reactions "one-pot" synthesis of 7, Carbon-based α-hydrobromination of 7 with Pyridinium tribromide gives 9,9 is asymmetrically reduced under the action of (+)-Ipc2BCl to obtain 11, which is then subjected to N-alkylation and sulfonamidation to generate (S)-3,4-dihydro-4-hydroxy-2-(3-methoxyl propyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide, the sulfonamide group was protected with trimethyl orthoacetate to give 15 , first introduce p-toluenesulfonyl group and then replace it with ethylamino group, and remove the sulfonamide group protecting group to obtain brinzolamide. The synthesis of intermediate 4 in this route is convenient, and each step of the reaction does not require column chromatography, and the total yield is 13.4%.Graphical Synthetic Routes of Olanzapine |
|
Indications |
Brinzolamide, a heterocyclic sulfonamide, is a topical CAI suspension that has a high affinity for the carbonic anhydrase II isoenzyme.Because the ocular hypotensive effect of the drug is equivalent whether dosed twice or three times daily, brinzolamide 1% may be administered twice daily. |
|
Therapeutic Function |
Antiglaucoma |
|
Biochem/physiol Actions |
Brinzolamide is a carbonic anhydrase II inhibitor used to lower intraocular pressure. |
|
Side effects |
Both brinzolamide and dorzolamide exhibit similar taste abnormalities. A single case report of the development of metabolic acidosis from topical brinzolamide has been described after twice-daily dosing. Other adverse events are negligible for brinzolamide except for some blurring of vision, attributable to its suspension vehicle. |
|
Veterinary Drugs and Treatments |
Brinzolamide is chemically similar to dorzolamide and reduces aqueous humor production by altering H+/Na+ active transport mechanisms associated with aqueous humor production in the ciliary epithelial cells. It can be used as a substitute for dorzolamide and some patients that exhibit excessive topical irritation following application of dorzolamide drops, tolerate brinzolamide better or vice versa. Cats seem to be particularly sensitive to irritation from topical dorzolamide and often brinzolamide can be used in these patients. Comparative data is available suggesting that brinzolamide and dorzolamide are equally effective in animal patients. |
|
Precautions |
Brinzolamide has the same contraindications and precautions as dorzolamide. |
|
references |
[1] desantis l. preclinical overview of brinzolamide. surv ophthalmol. 2000 jan;44 suppl 2:s119-29. |
|
Definition |
ChEBI: Brinzolamide is a sulfonamide and a thienothiazine. It has a role as an antiglaucoma drug and an EC 4.2.1.1 (carbonic anhydrase) inhibitor. |
|
Brand name |
Azopt (Alcon). |
|
General Description |
Brinzolamide is a small molecular weight compound that has an ability to bind melanin. This drug is used in ocular therapy. |
InChI:InChI=1/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1
The invention discloses a method for pre...
The invention discloses preparation meth...
Solid phase extraction (SPE)-chiral sepa...
The present invention discloses the phar...
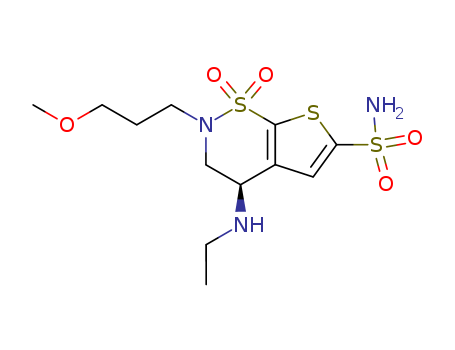
C21H29N3O8S4

brinzolamide
| Conditions | Yield |
|---|---|
|
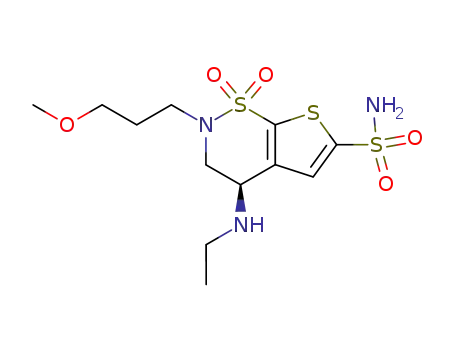
With sodium thiomethoxide; In methanol; at 0 - 25 ℃; for 12h; Reagent/catalyst;
|
83% |
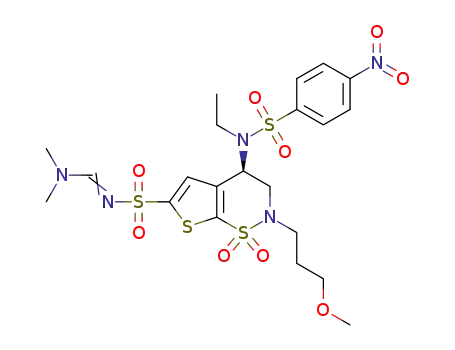
C21H29N5O9S4


brinzolamide
| Conditions | Yield |
|---|---|
|
With sodium tetradecyl mercaptan; In methanol; at 0 - 25 ℃; Reagent/catalyst;
|
81% |
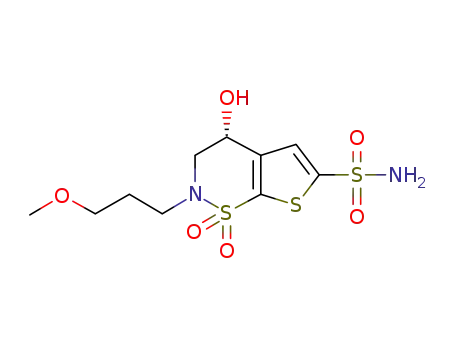
(R)-3,4-Dihydro-4-hydroxy-2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide 1,1-dioxide
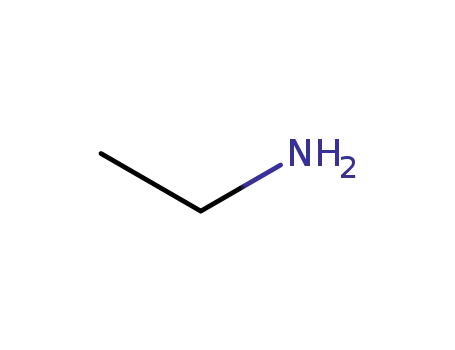
ethylamine
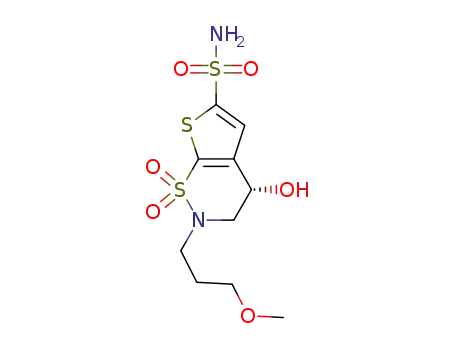
(S)-3,4-dihydro-4-hydroxy-2(3-methoxypropyl)-4H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide
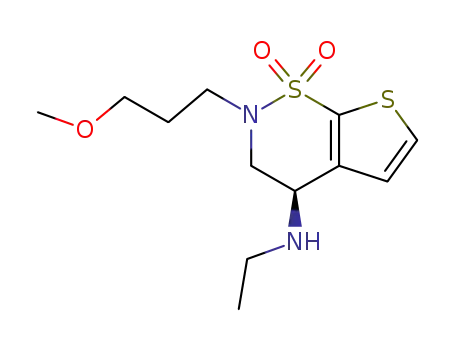
(R)-3,4-dihydro-4-(N-ethylamino)-2-(3-methoxy)propyl-2H-thieno[3,2-e]-1,2-thiazine-1,1-dioxide
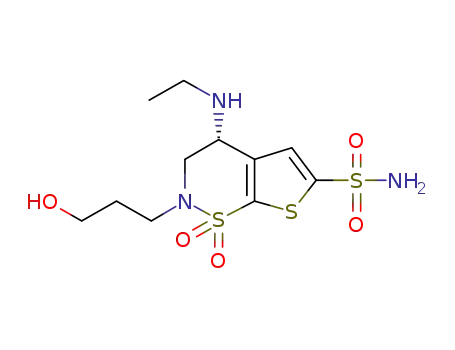
(R)-4-(ethylamino)-2-(3-hydroxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide1,1-dioxide
